Abstract
Background and Objective: Romiplostim, is a TPO agonist approved for the treatment for adult ITP. It follows a weight-based and platelet response-guided dose titration algorithm to maintain patients' platelet counts (PC) between 50 and 200x10 9/L, after which a maintenance dose is administered once weekly to keep platelet counts within the target range. While self-administration of romiplostim maintenance dose has been approved in EU for adult patients, in the US romiplostim is only approved for weekly administration by a healthcare provider (HCP) using an 0.01 mL graduation syringe to ensure dosing accuracy; a dose tolerance margin of romiplostim has never been investigated before. The following work explores the impact of dosing tolerance on PC to assess the feasibility of romiplostim self-administration in adult ITP patients.
Methods: The analysis was based on a previously developed pharmacodynamic model of romiplostim in ITP patients (Perez-Ruixo et al, 2012, Gibiansky et al, 2021). Previous romiplostim clinical studies in patients with ITP were leveraged to develop an updated model of 475 ITP patients (>50 kg, weekly romiplostim total dose > 100 µg and between 1 and 10 µg/kg stable maintenance dose for at least 1 month, self-administration) and assess potential differences in PC between ITP patients who qualify for self-administration and the general adult ITP population. Comparison of observed and model predicted PC and romiplostim dose over time confirmed the adequacy of the model to assess the impact of varying dose tolerances on PC that may occur with self-administration.
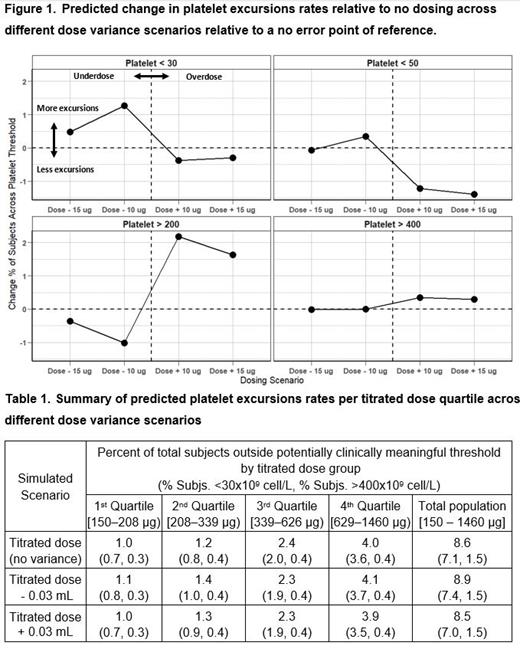
Under the proposed guidelines for romiplostim self-administration eligibility, an adult patient would self-administer up to 4 weekly stable maintenance doses of romiplostim between two platelet measurements 1 month apart. To evaluate the impact on PC over 1 month when patients self-administered all four weekly doses versus patients receiving the same prescribed dose by a healthcare provider, three dosing scenarios were considered: (1) exact titrated dose (no dose deviation), (2) titrated dose plus 0.03 mL or 15 µg of romiplostim, and (3) titrated dose minus 0.03 mL or 15 µg of romiplostim. To further characterize the number of ITP patients with clinically meaningful platelet deviations, the predicted number of virtual patients with a PC below 30 x10 9/L or above 400 x10 9/L were also summarized for each simulated scenario.
Lastly, the simulated population was divided into four groups based on the magnitude of their prescribed dose and used to evaluate whether the prescribed romiplostim dose influences platelet excursion rates following the investigated dose scenarios.
Results: Simulations suggest that 4 successive weekly doses of ± 0.03 mL (or ± 15 µg) the prescribed romiplostim dose is unlikely to cause a significant change in platelet excursion rates relative to continuous administration of the exact prescribed dose. No meaningful increase (0.1 - 0.4%) was observed in the predicted excursion rates across these thresholds for either the under- or overdosing scenarios (Figure 1).
This results also show that while there is a slight trend between the magnitude of the prescribed dose and the fraction of subjects that experience a platelet excursion, it does not result in a meaningful increase (<4%) in platelet excursions in any of the evaluated subgroups. Specifically, we observed minimal changes in the fraction of patients with a platelet excursion outside the potentially clinically meaningful thresholds across all simulated dose groups and dosing scenarios (Table 1).
The results from this analysis suggest that possible dosing variance expected from the self-administration of romiplostim by an eligible lay person (± 0.03 mL or 15 μg) with monthly PC evaluation will not lead to a significant increase in the rate of platelet count excursions for patients who achieve a stable romiplostim dose and meet the proposed eligibility criteria.
Serrano Castillo: Amgen: Current Employment, Current equity holder in publicly-traded company. Saad: Amgen: Current Employment, Current equity holder in publicly-traded company. Wang: Amgen Inc: Current Employment, Current equity holder in publicly-traded company. Zhang: Amgen: Current Employment, Current equity holder in publicly-traded company. Chow: Amgen: Current Employment, Current equity holder in publicly-traded company. Doshi: Amgen: Current equity holder in publicly-traded company; Amgen: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal